When you access Maptrial, you will find all the blank monitoring reports for your visits.
The content of these reports is based on the templates provided by the study sponsor.
Cloning a Monitoring Report
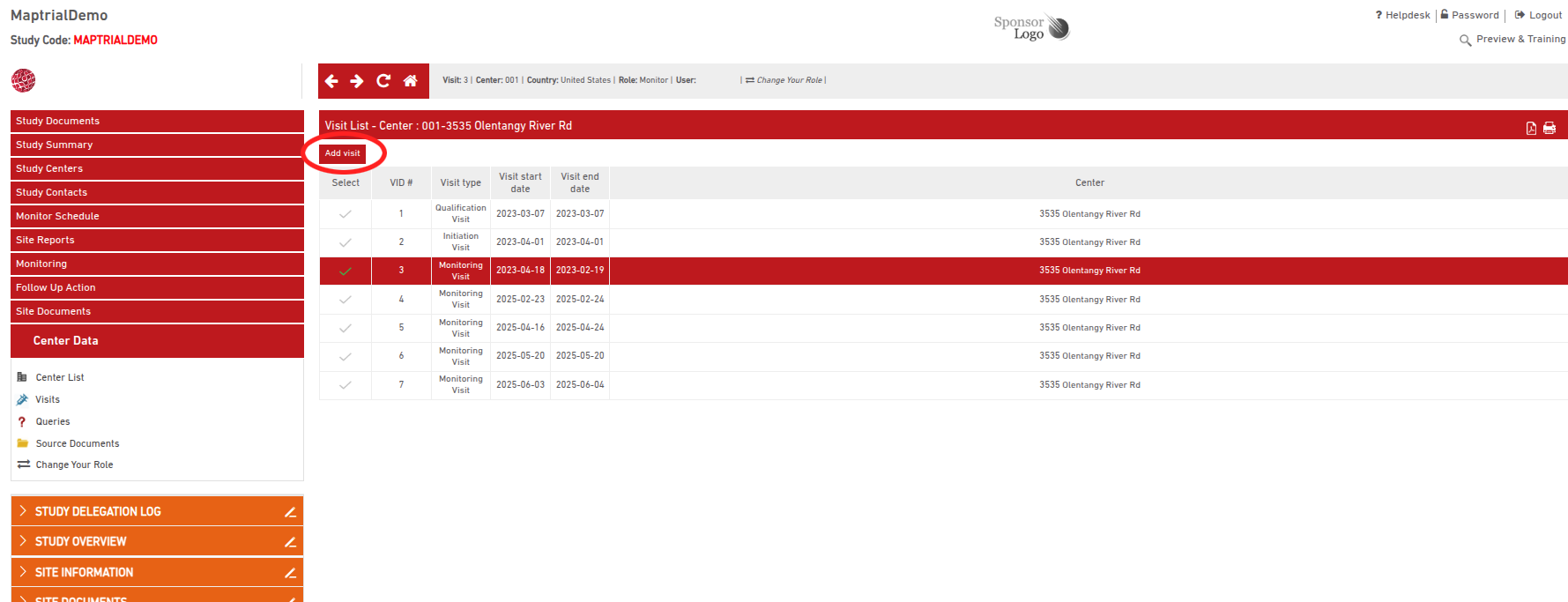
When a CRA creates a new visit, they can choose to retrieve key data (including open follow-up actions) from a previous visit—typically the latest one—to facilitate faster data entry.
Based on the sponsor specifications for the Maptrial setup, only “FUp New/Ongoing” are copied, but additional data can be transferred upon Sponsor request and feedback.
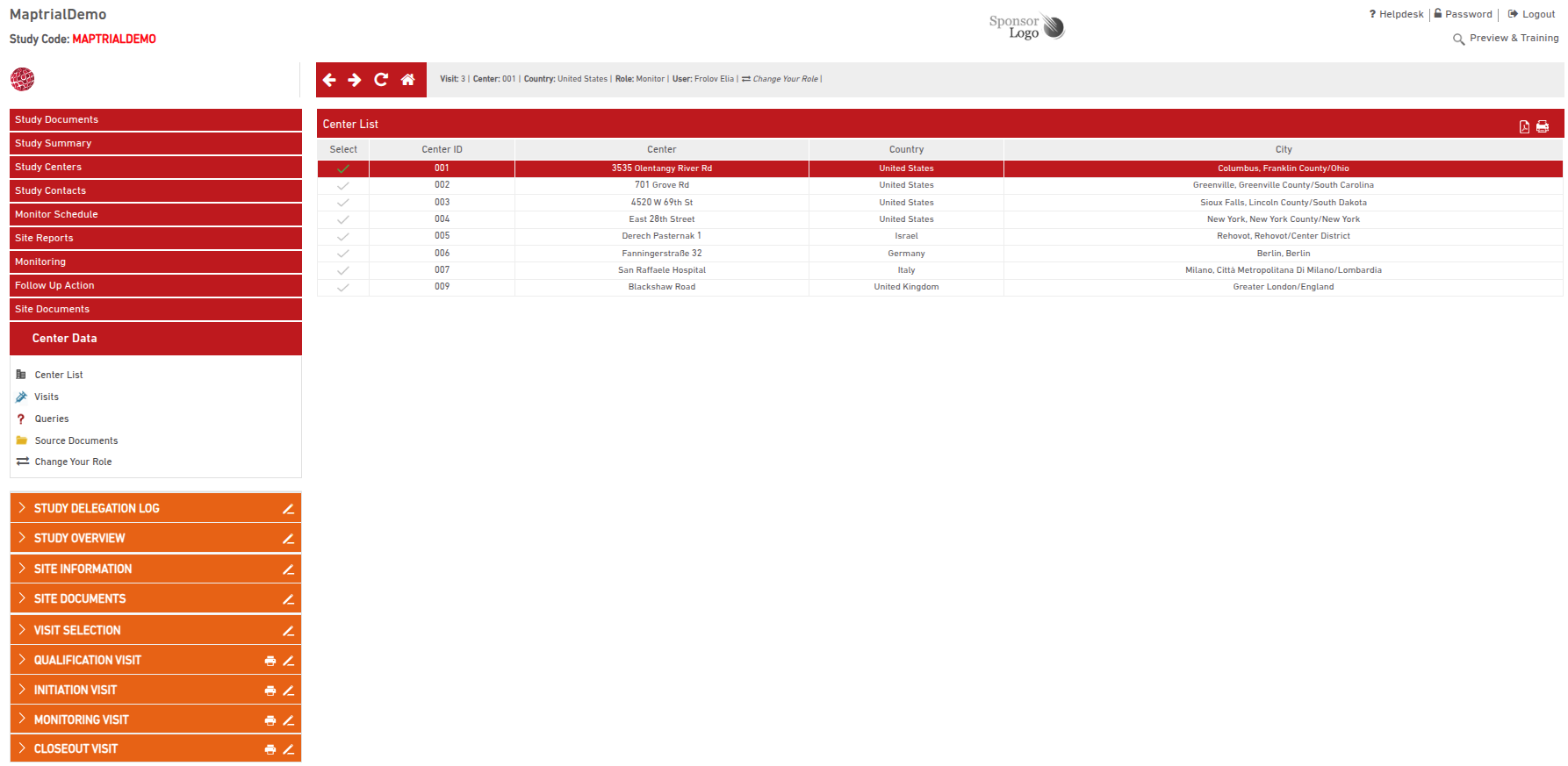
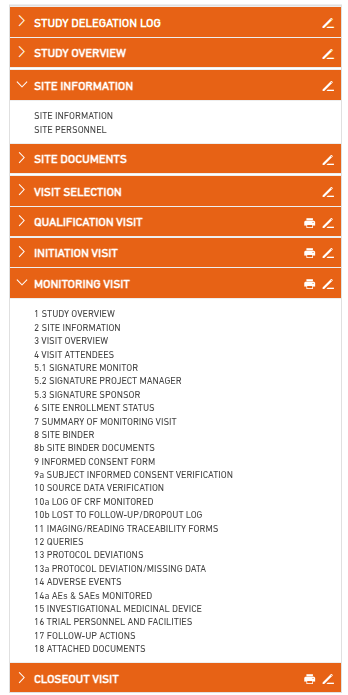
Monitoring visit reports are structured into multiple sections based on your provided templates. Access to each site is role-based—users can only view and manage reports for sites assigned to them.
After selecting a site and an existing visit (e.g., an interim monitoring visit), the corresponding report opens with all previously entered data.
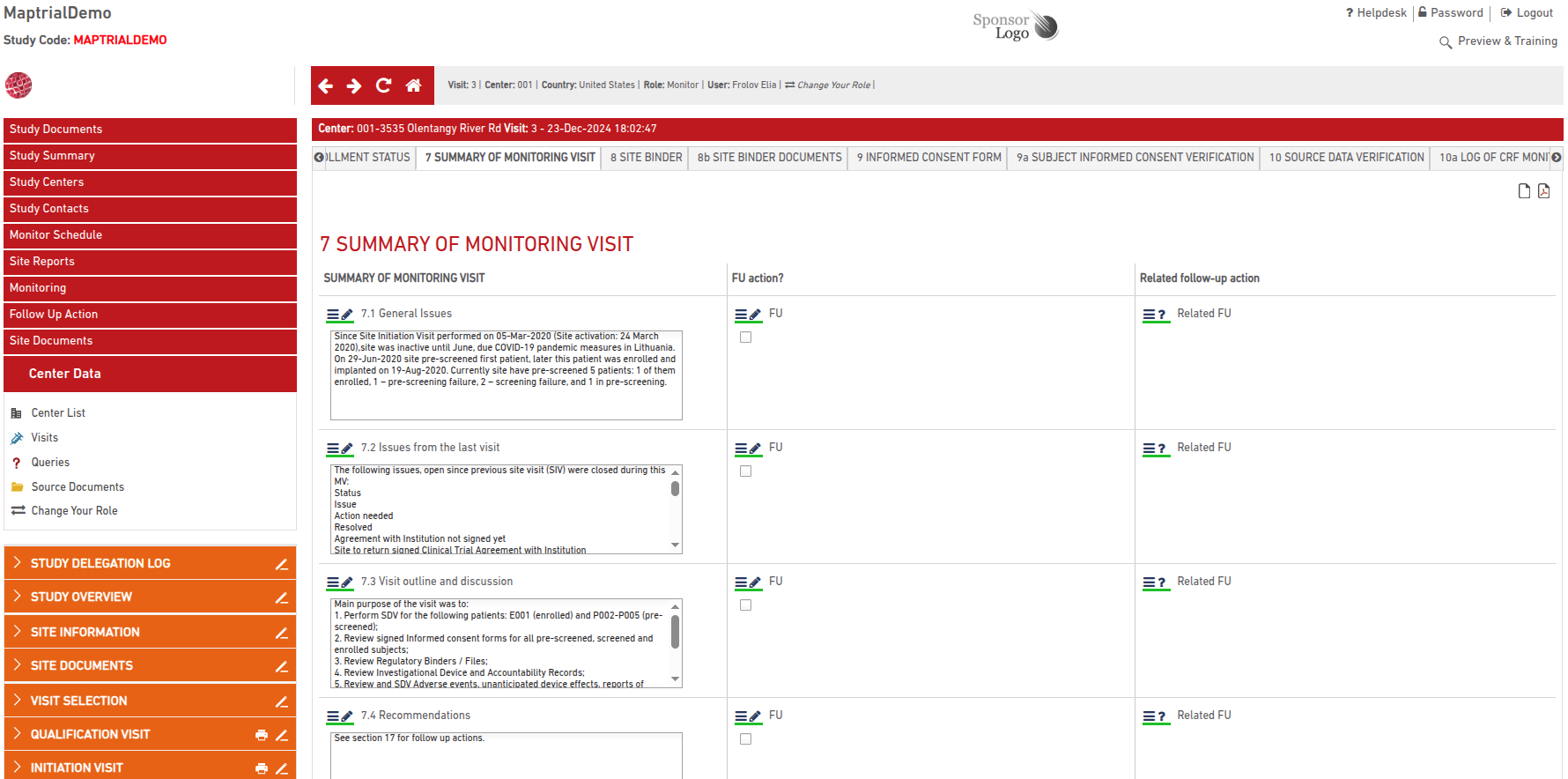
These reports are organized in the same structure as the original templates and can be navigated manually section by section.
If the report has already been signed, signature details are displayed and tracked.
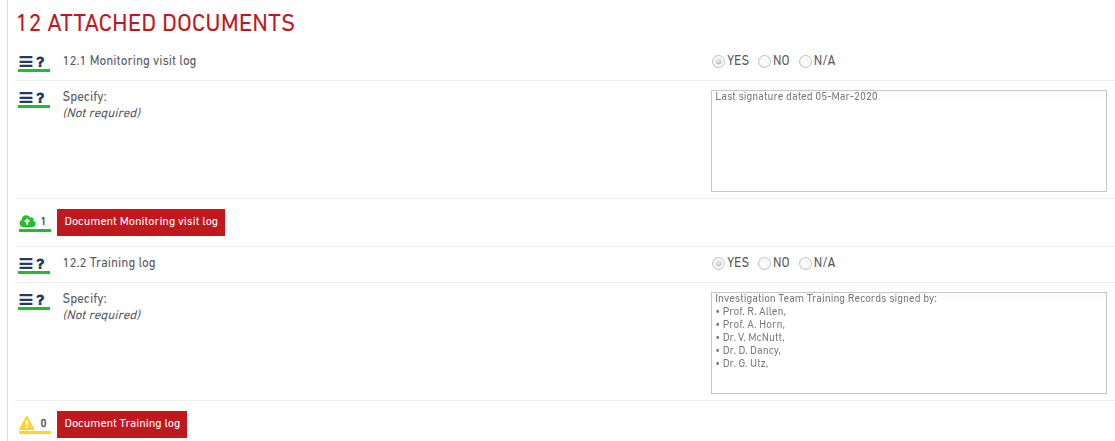
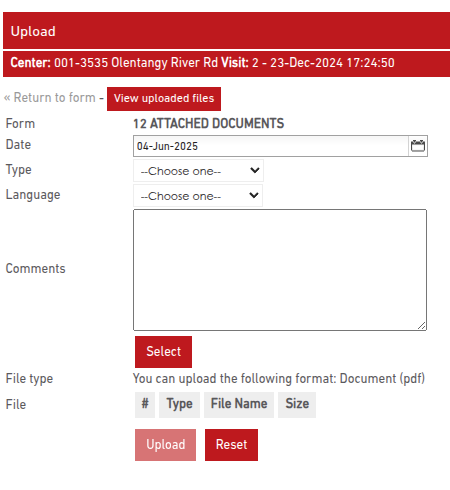
You can upload and manage Attached Documents directly within the monitoring visit report. Use the upload button to upload PDF files relevant for the monitoring visit.
A visual indicator shows upload status:
- Yellow icon: No documents uploaded
- Green icon: One or more documents uploaded
- A number next to the icon indicates how many documents are attached
To upload a document:
- Click the upload icon in the appropriate section.
- On the upload page, click Upload File.
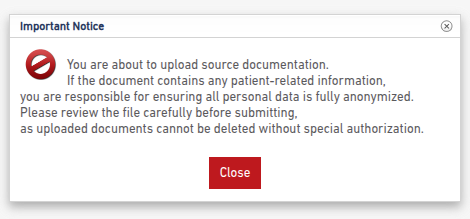
- Confirm that the file is redacted and does not contain PHI (Protected Health Information) per HIPAA (U.S.) or GDPR (EU) regulations.
- Select a PDF file, enter the upload date, and click Upload.
Once uploaded, the icon turns green and the document counter updates. Multiple files can be uploaded per section.
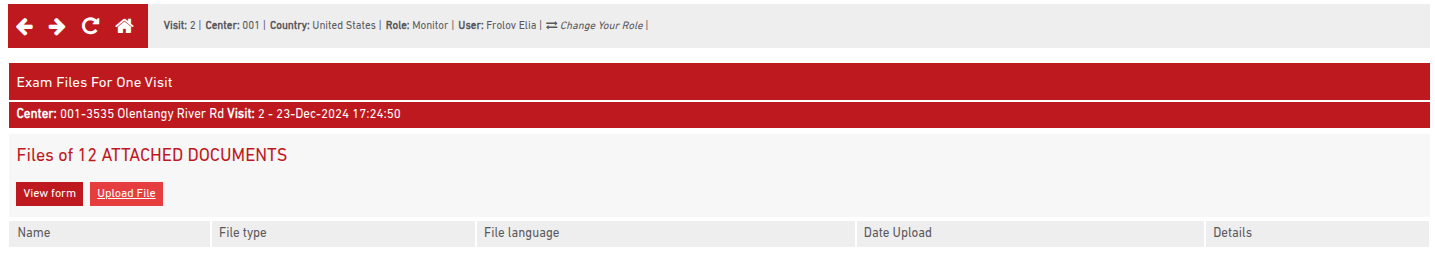
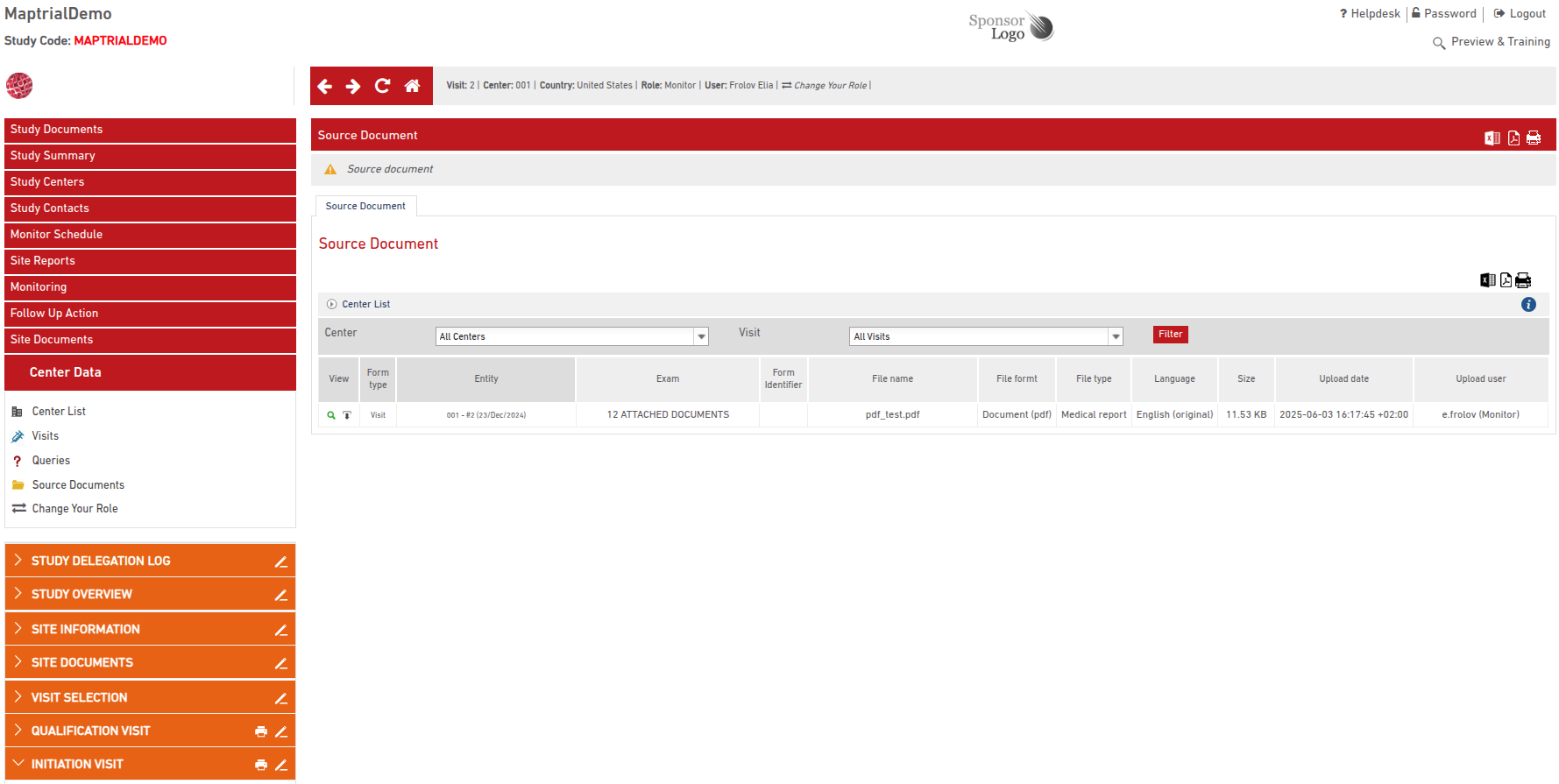
All uploaded documents appear in a centralized Document Library. This list includes:
- Site and visit details
- Document section
- File name
- Upload date
- Uploading user
Documents can be downloaded at any time with a single click.
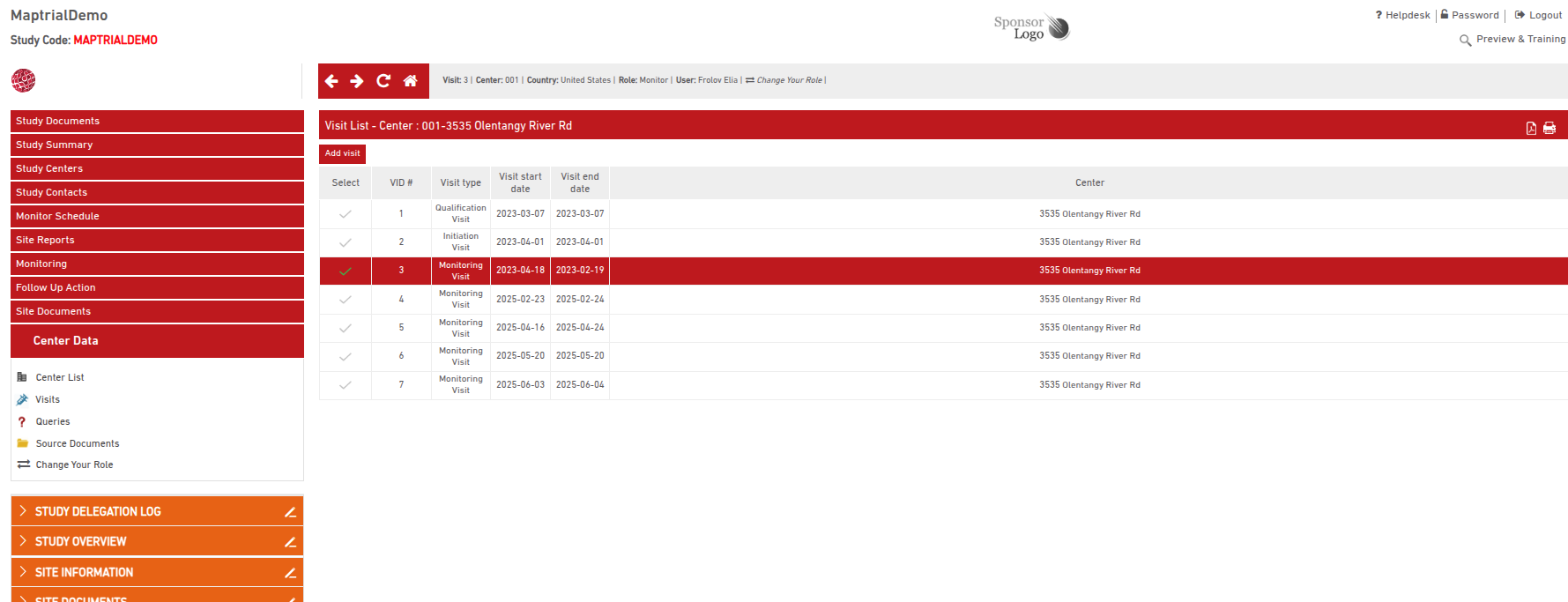
How to Manage Previous Reports
- Select the previous report;
- Edit or review : monitor can modify the report; Sponsor can create queries
- Sign and Lock Completed Reports
Select a specific visit—such as a Site Qualification Visit Report—form the list.
This allows you to open and review the forms completed by the monitor, providing an initial view of how to navigate and interact with Maptrial.
Select the visit forms:
- Monitor can update a value by modifying the field content and saving the form.
- If required, Sponsor can add a query by clicking on the “Query” icon and typing the query text.
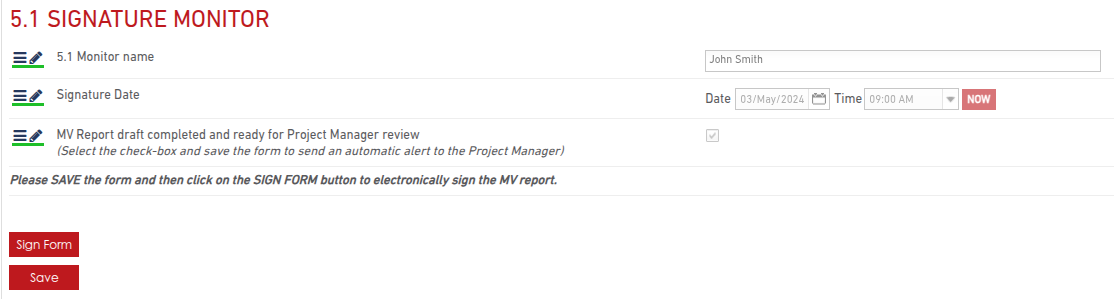
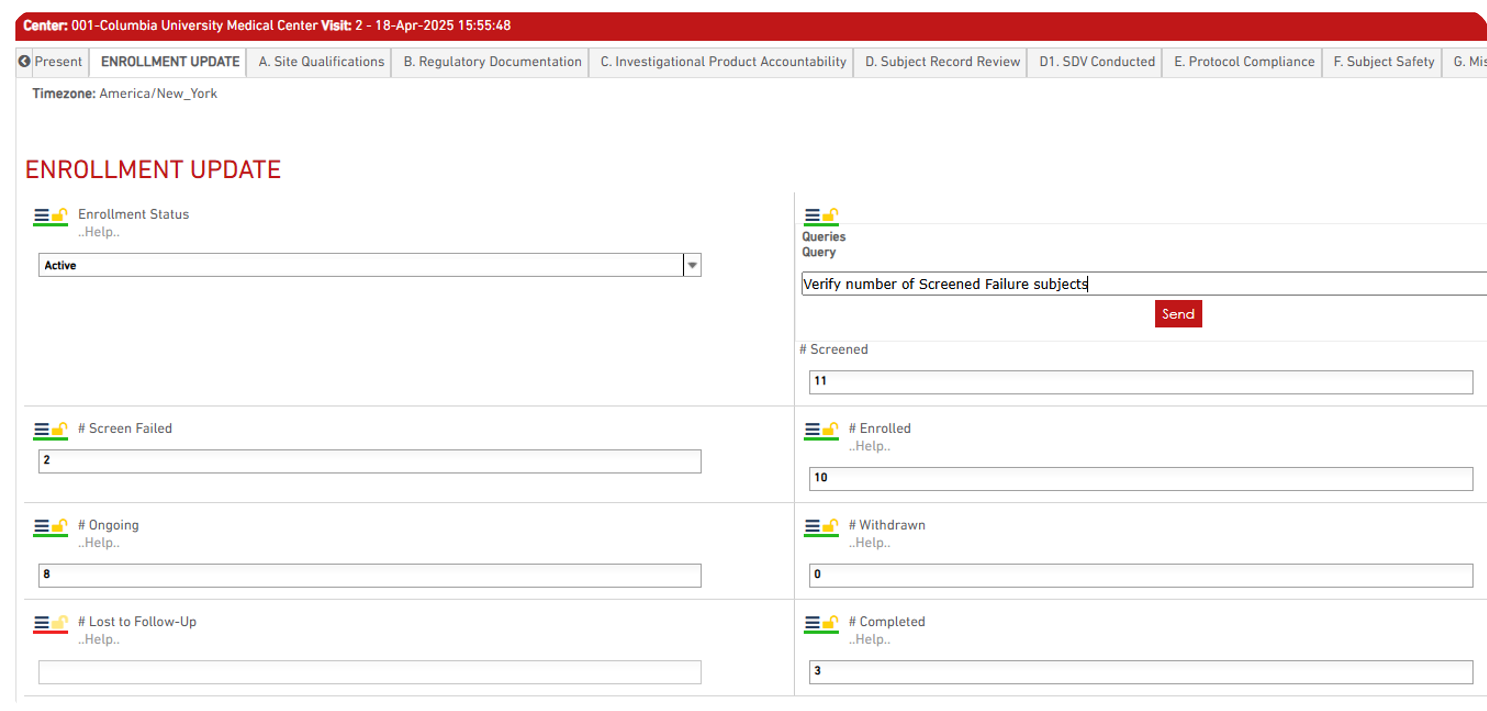 Once the MV report is complete:
Once the MV report is complete:
- Monitor reviews and signs off the report.
- Sponsor then reviews and signs the report. Then Sponsor has the ability to lock the form, finalizing the report.